Quick Answer (TL;DR)
In the Philippines, cosmetic products (like skincare, rejuv sets, whitening creams) are regulated differently from drugs:
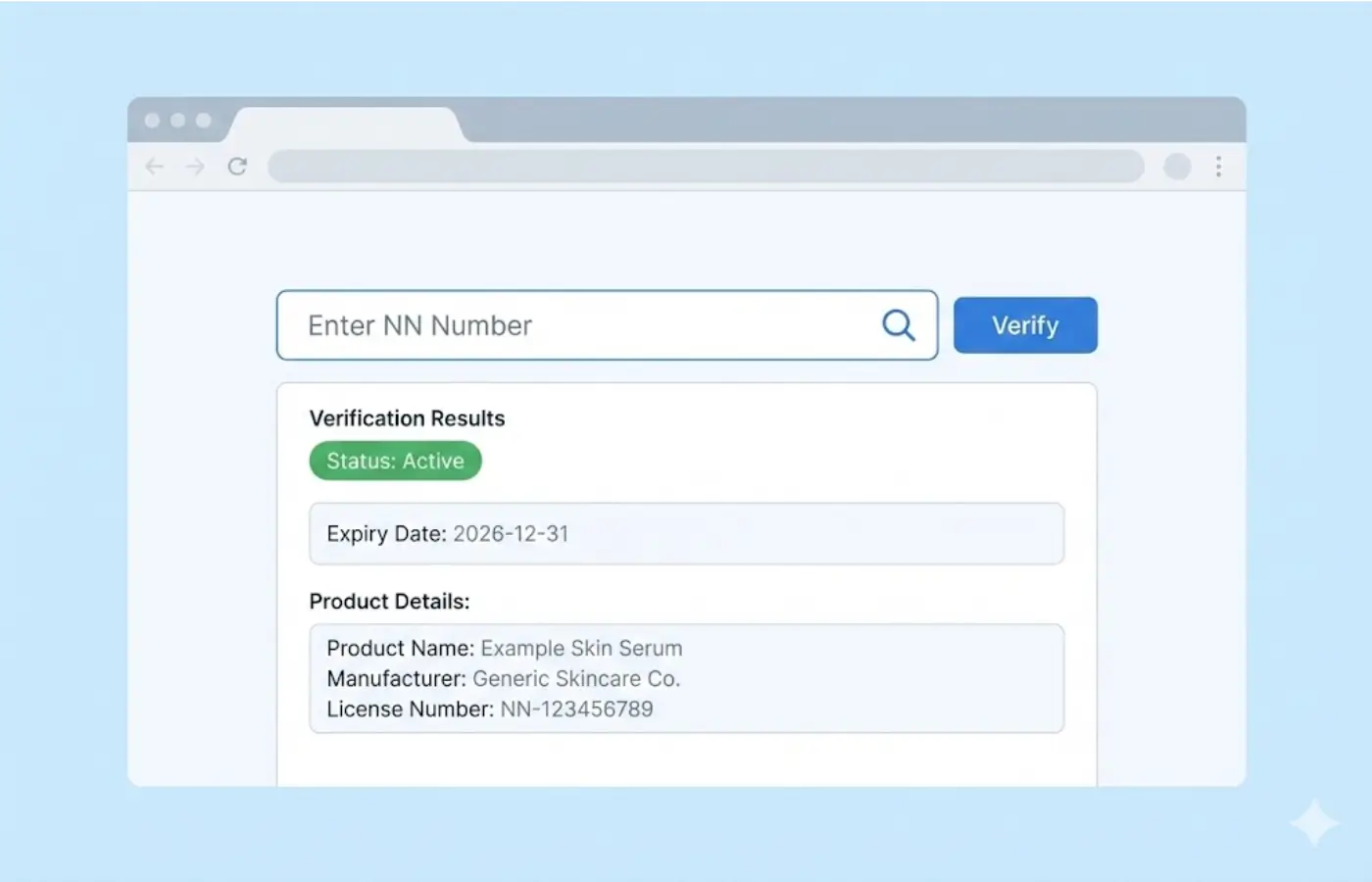
- NN (Cosmetic Product Notification) = the product has been notified to the FDA per SKU
- LTO (License to Operate) = the establishment/manufacturer has a license to operate
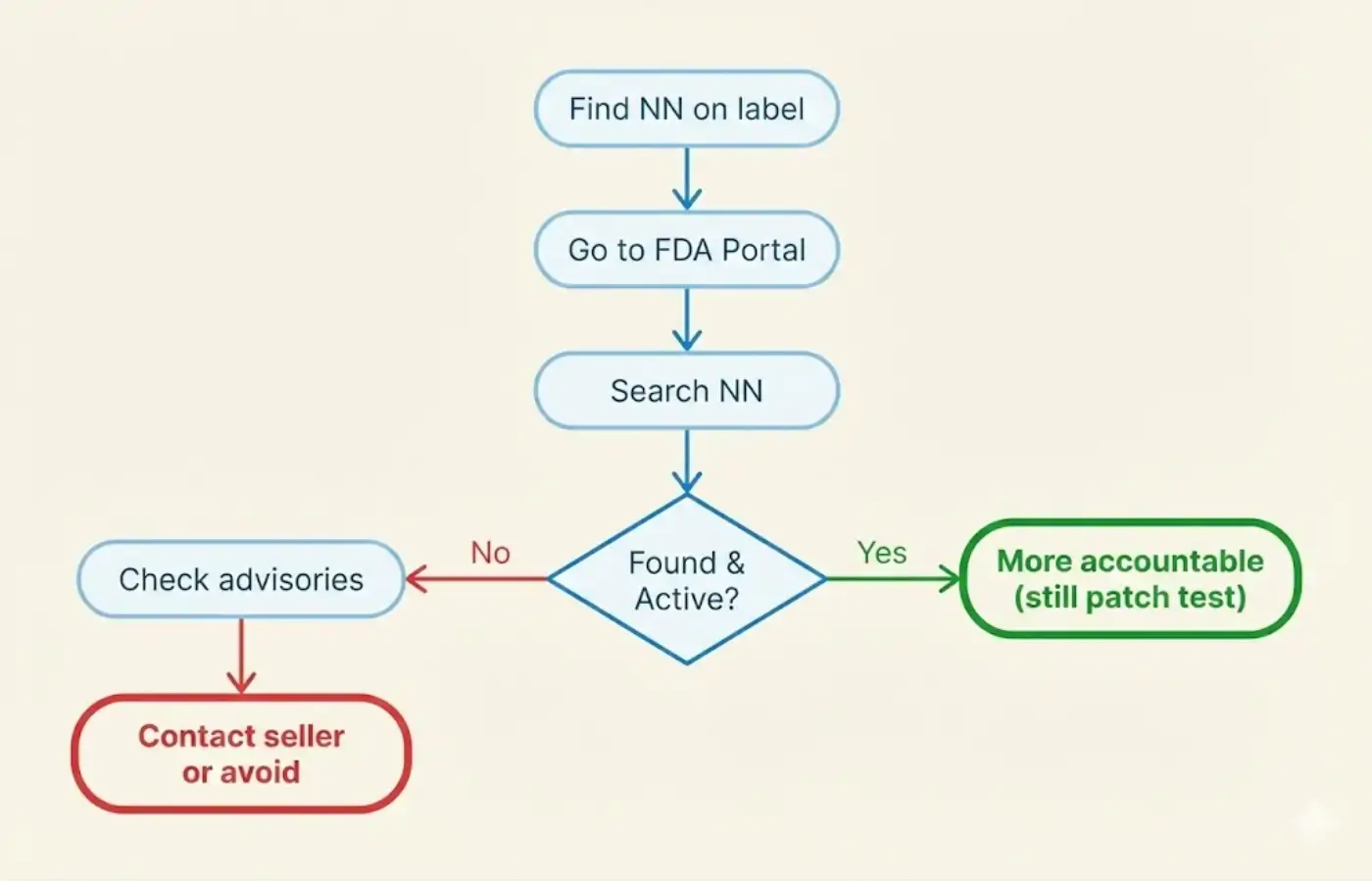
Why verify?
- Unnotified cosmetics may contain undeclared ingredients (including banned substances)
- The FDA has issued multiple advisories against unauthorized cosmetic products [5][6]
- Verification protects you from fake, adulterated, or unsafe products
Portal: verification.fda.gov.ph [1]